| |
 |
| |
 |
| |
| 1 Definitions of Terms |
| 1-(1) Ao: Theoretical Air Amount (= Oxygen Amount/21%) |
| 1-(2) Gow: Theoretical Wet Exhaust Gas Amount (Inside Furnaces) |
| 1-(3) God: Theoretical Dry Exhaust Gas Amount (After Atmospheric Dissipation) |
| 1-(4) Higher Heating Value (Inside Furnaces) |
| 1-(5) Lower Heating Value (Net Heating Value, After Atmospheric Dissipation) |
| 2 Database for Gaseous Fuel |
| 3 Sulfur Content |
| 3-(1) Chemical Equilibrium Ratio of SOX |
| 3-(2) Sulfuric Acid Corrosion of Equipment |
| |
 |
| 1 Definitions of Terms |
| |
| Gaseous fuels include hydrogen, propane gas, natural gas, and etc. Most gaseous fuels are mixture of flammable gas elements. The calorific value, amount of exhaust gas, air required for combustion, and so on, are determined for each element. The values as a fuel is proportional to the mixing ratio. |
| |
| The amount of oxygen required for combustion and that of exhaust gas can be determined by the coefficients of the chemical equation for each element. And then, the amount of air required for combustion can be calculated from the amount of oxygen. Let's pay attention that chemical formulas are expressed in moles, not weight or volume. For the gases, all gases occupies same volume for same mole, therefore, we can consider that the number of moles as volume ratio. |
| |
 |
| 1-(1) Ao: Theoretical Air Amount (= Oxygen Amount/21%) |
| |
| The theoretical amount of air is the amount of oxygen required in the chemical equation for combustion divided by the oxygen content of the air. The author thinks the oxygen content should be twenty one percent (21%); however, the exact oxygen content is twenty point nine five percent (20.95%). |
| |
| Ao = oxygen mole coefficient / atmospheric oxygen concentration |
| |
 |
| 1-(2) Gow: Theoretical Wet Exhaust Gas Amount (Inside Furnaces) |
| |
| When huydrogen burns, it turns into water (water vapor), and the amont of wet exhaust gas means the amount that includes the water vapor. Industrially, this is the situation inside a heating furnace. When cooking on gas stove at home, the state is the same. |
| |
| Gow = Ao + Coeffect of CO2 + Coefficient of H2O - Coefficient of O2 |
| |
 |
| 1-(3) God: Theoretical Dry Exhaust Gas Amount (After Atmospheric Dissipation) |
| |
| After the combustion gases are used in wet condition in the furnace, they are released into the atmosphere and their temperature decreases to atmospheric one. The water vapor produced by combustion should return to liquid water, and the exhaust gas remaining in the environment is the value excluding water vapor. This is called the dry gas amount. Therefore, the value is the amount of wet exhaust gas minus water vapor. |
| |
| God = Gow - Coefficient of H2O |
| |
 |
| 1-(4) Higher Heating Value (Inside Furnaces) |
| |
| Since the homepage is about thermodynamics, the author would like to deal with the amount of heat generated when combustion gas does work. It is the calorific value when the exhaust gas is wet, and is called the higher calorific value. |
| |
 |
| 1-(5) Lower Heating Value (Net Heating Value, After Atmospheric Dissipation) |
| |
| After the heating and other tasks are completed, the exhaust gases are released into the atmosphere, and the water vapor is removed. The calorific value of the dry exhaust gas is called the lower heating value, or net heating value. We do not use it in thermodynamics, so let's omit it here. |
| |
 |
| 2 Database for Gaseous Fuel |
| |
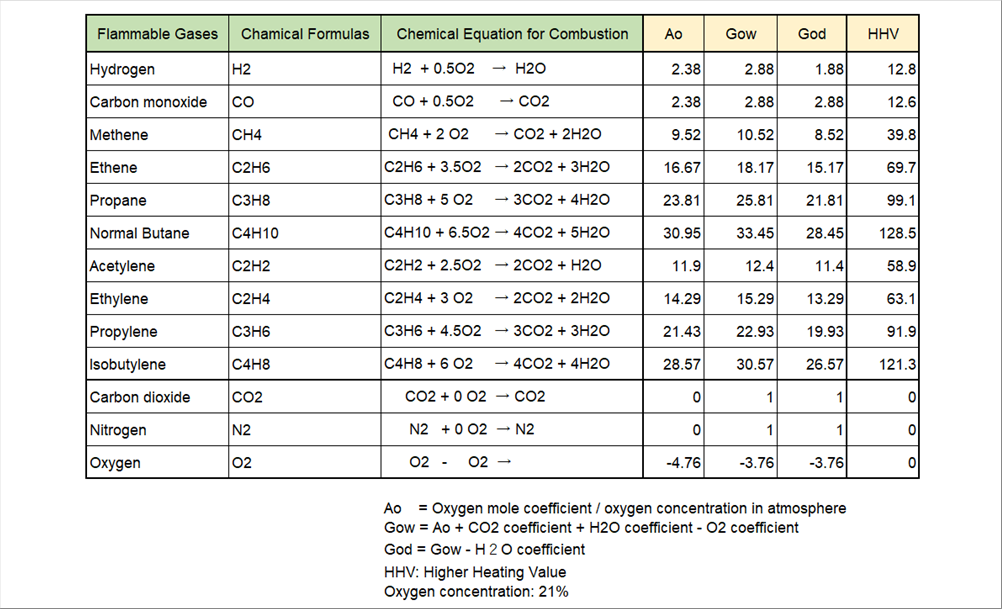
| The table below is a database of air required for combustion Ao, theoretical wet exhaust gas coefficient Gow, theoretical dry gas coefficient God, and high heating value of major combustion gas elements. For some manufacturing reasons, carbon dioxide, nitrogen, and oxygen may be included, so they are also included for reference only. |
| |
 |
| |
| Since cargon dioxide and nitrogen do not burn, the theoretical air amount is zero (0), but since they become exhaust gas, the coefficient of exhaust gas volume is one (1), |
| |
| The sign of oxygen is negative, because the oxygen in gaseous fuels can be used for combustion, so the same amount of air can be omitted. |
| |
 |
| 3 Sulfur Content |
| |
| Gaseous fuels may contain trace amount of sulfur S. Sulfur content burns and becomes sulfur oxide SOX. |
| |
| The main components of SOX are sulfur dioxide SO2 and sulfur trioxide S3. The ratio changes depending on the temperature. This is called the "equilibrium state." Although it is an interesting phenomenon, the sulfur content is so small that it has little effect on thermal calculations. |
| |
| However, sulfur oxide (SOX) is a very important element from the viewpoint of air pollution prevention and sulfur acid corrosion of heat exchangers in heating furnaces and heat treatment furnaces. |
| |
 |
| 3-(1) Chemical Equilibrium Ratio of SOX |
| |
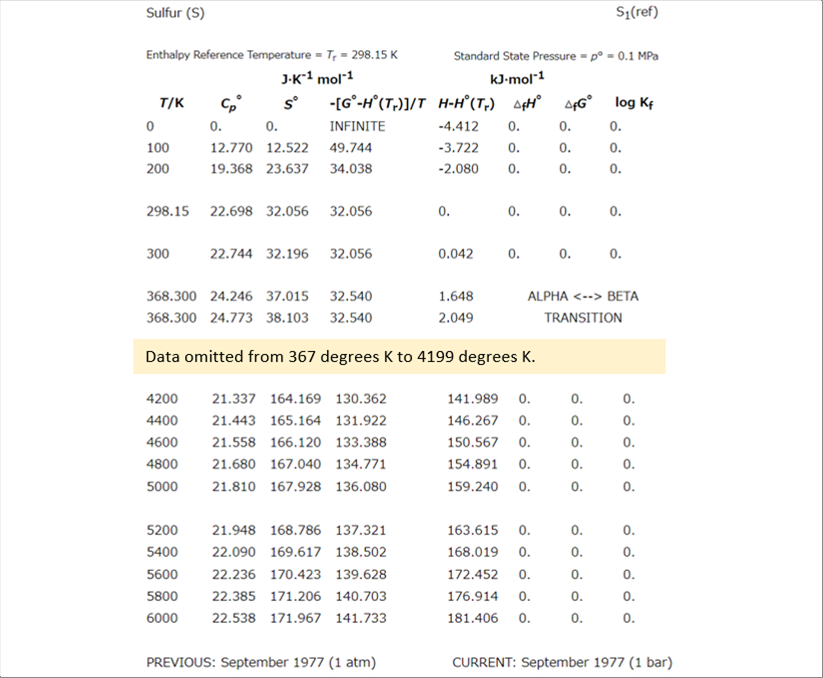
| For the chemical equilibrium of sulfur, the author referred to "NIST - JANAF Thermochemical Tables." NIST : National Institute of Standards and Technology, U.S. Department of Commerce |
| |
| In conclusion, the main component of SOX is SO3 at room temperature and SO2 at high temperature. |
| |
| NIST data is provided as a text tables, so we can manage it in Excel, etc. for calculations. The temperature range if from absolute zero to sis thousand (6 000) degrees K., the following table shows the first and last part. |
| |
 |
| |
| |
| Using the data, we can calculate SOX by determining the chemical equilibrium ratio between SO2 and SO3. |
| |
| Chemical equilibrium is not the main topic of our discussion, so let's omit it here. However, we can calculate the equilibrium equation between SO2 and SO3, because three (3) equilibrium equations are known: one is an equilibrium equation for S, O2, and SO3, the second one is for S, O2, and SO2, the third one is for SO2, O2, and SO3. |
| |
| K = Kf[SO3] / Kf[SO2] |
| |
| K = 10 ^ (logKf[SO3] - logKf{SO2]) |
| |
| Out of NIST data, we will use data on the temperature range used in heating furnaces and in heat treatment furnaces. |
| |
 |
| 3-(2) Sulfuric Acid Corrosion of Equipment |
| |
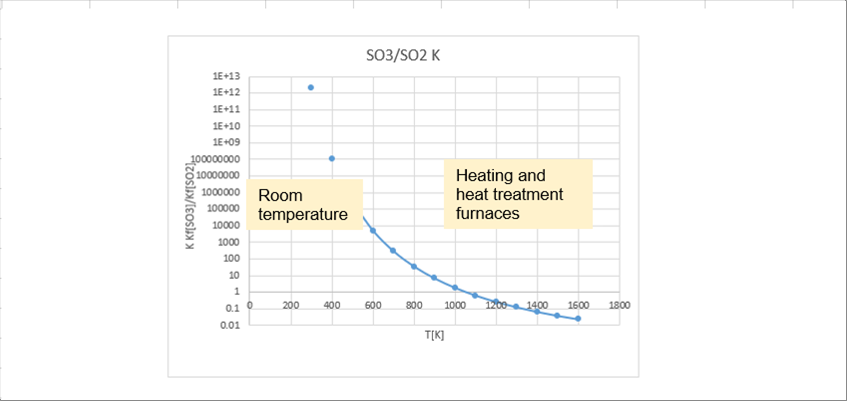
| The following graph shows the temperature range form room temperature to the temperature range used in heating furnaces ans heat treatment furnaces. |
| |
 |
| |
| As seen in the graph, the authors say that the total amount of SOX is SO3 at room temperature. Next, let's take a look at the situation inside the heating furnaces and heat treatment furnaces. In the temperature range inside the furnaces, SO3 degreases rapidly. It is because SO2 increases rapidly; at seven hundred (700) degrees C (1100K), it becomes half and half, and at 1 200 degrees C (1 500K), about 90% is SO2. |
| |
| We found that there is a lot of SO3 in the low temperature section of the exhaust gas heat recovery equipment. When S3 dissolves into water, it becomes sulfuric acid, so sulfuric acid corrosion is thought to progress in low-temperature sections. |
| |
| |
| Author: T.Oda |
| The page was prepared in Excel, and html and css files were automatically generated by the excel2web. |





























