| |
 |
| |
 |
| (1) Introduction |
| (2) Burning Flame |
| (3) Compositions of the Fuel Gas |
| (4) Heating Values of Gases (Higher Heating Value) |
| (4)-1 Heating Values, Natural Gas |
| (4)-2 Which of Higher or Lower Heating Values (Net Calorific Value) for Simulation? |
| (4)-3 Sulfur in the Fuel |
| (5) Theoretical Air Volume, Theoretical Exhaust Gas Amount |
| (5)-1 Air-Fuel Ratio |
| (6) Is the Forced Convection Heat Transfer Necessary? |
| (7) Chemistry of Combustion |
| |
 |
| (1) Introduction |
| |
| The convection heat transfer exist but varies due to the variation of the velocity difference between the combustion gas and the material to be heated. There are two (2) kinds of the convections, one is the natural one and another forced one. The natural convection shall be used, because it is literary naturally happening at any time. How about the forced one? The combustion gas flows from the combustion burners up to the chimney through the inside of the furnace. Therefore, the speed of the combustion gas is fast if the cross section of the furnace is narrow and the quantity of the combustion gas is big. Please note that due to the viscosity of combustion gas, the speed of the gas is the fastest in the middle of the roof and the floor, and zero (0) at the inside surface of the furnace. It is assumed that when the materials to be heated are charged close to the floor, for example, it may ne negligible small. It may be rare to start the temperature simulation from the combustion of the fuel gas; however, it is important to understand the combustion and the velocity of the combustion gas. |
| |
| Please find the fuel composition, calculation of the heat generation according to the fuel-air ratio. We can obtain the average combustion gas speed if we divede it by the area of the cross section. |
| |
 |
| (2) Burning Flame |
| |
| There are three (3) kinds of fuel, which are the solid one like coals, the liquid one like oil, and the gas one like hydrogen and natural gas. Let's focus on the gas fuel here, |
| |
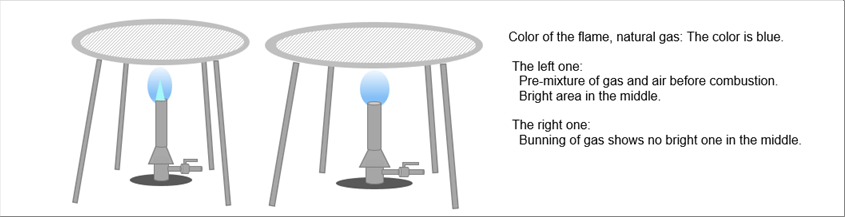
| The following pictures show the burning flames of the natural gas on the burners, where we see the blue flame. If the air is mixed with air in advance by opening a air vent, we see a bright area in the middle of the flame, no bright area otherwise. |
| |
 |
| |
| The main component of the natural gas is methane. It comes to our mind that the difference of the color might be due to the difference of the burning processes of methane. For your reference, the same color difference is observed when the gas is propane. The processes of the propane are also similar to that of methane. |
| |
| The combustion process of the heating furnace for the steel materials is same as the above picture. One of the differences between the heating furnace and the burners is the sealability. The heating furnces show higher sealability than that of the buners, and therefore, the right side of the combustion, which use the air around the burners, rarely happen. |
| |
| The color of the flames and pre-mixture method are out of scope here. Let's focus on the calorific values and the products of the combustion. |
| |
 |
| (3) Compositions of the Fuel Gas |
| |
| The gas fuel consists of methane, propane, and other flammable gases. The components and the ratio are very important for the calculation of the calorific value and the products. The fuel gas on the market contains nonflammable one and oxygen as well. All of them are necessary for the calculation. |
| |
| It is well know as the flammable gases such as hydrogen, methane, propane, and so on. Pleaser remind that the carbon monoxide is one of the flammable gases. The hydrogen generates water, the carbon monoxide produces carbon dioxide. The rest of the flammable gases consists of carbon and hydrogen, and all of them produces the carbon dioxide and vapor, which turns into liquid water when it cools. |
| |
 |
| |
| The carbon dioxide and nitrogen are nonflammable gas. Please note that the oxygen do not burn but is used for the burning. Therefore, the oxygen in the fuel gas reduces the necessary air for the burning. |
| |
 |
| (4) Heating Values of Gases (Higher Heating Value) |
| |
| The main components of the generally used fuel gas, such as the natural gas and the propane gas, are carbon and hydrogen. They are oxidized by the oxygen, and during the oxidization process, heat, carbon dioxide and vapor are produced. |
| |
 |
| |
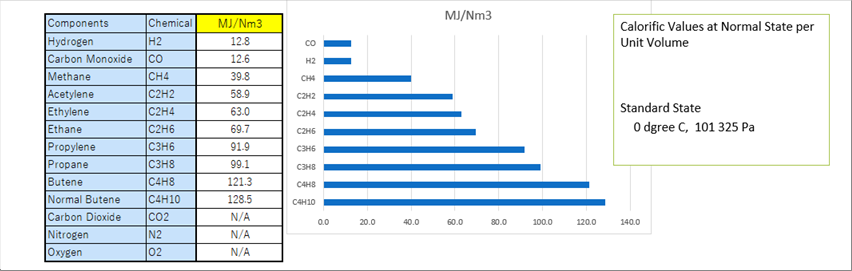
| The above table and picture show the calorific values at the normal state per unit volume. Some of you might be surprised that the calorific value of carbon oxide is same as that of hydrogen. As seen, propane can produce about nine (9) times of hydrogen. |
| |
| The caloric value per a unit volume of each component is already measured and is found constant. Therefore, if we know the ratio of each component, we can calculate the calorific value of the mixed gas. |
| |
 |
| (4)-1 Heating Values, Natural Gas |
| |
 |
| |
| According to the website of Tokyo Gas show the components and the calorific value of the 13A as above. (Translation by the author, who owes any mistake.) |
| |
| When I substitute the components to the unit calorific values table, introduced before, the total values comes to forty for point 8 (44.8) MJ. It is very close to the website one, 45 MJ. |
| |
 |
| (4)-2 Which of Higher or Lower Heating Values (Net Calorific Value) for Simulation? |
| |
| Please find the definitions of both values. |
| |
| Higher Heating Value: Heating value just after the combustion |
| |
| Lower Heating Value: Heating Value when it cools before the combustion (True Heating Value) |
| |
| The lower heat value is also called the true heating value, and therefore, I tool it the one when I negan leaning. However, we shall take the higher one for the simulation. It is due to the following reasons. |
| |
| The fuel produces heat as the carbon dioxide and the vapor at high temperature. The heat at the moment is the higher heating value. The calorimeter measures it. We can utilize the heat (energy) for the turbines of the thermal power plant, for the engines of the motorcycles, heating materials in the heating furnaces. |
| |
| After such work, the carbon dioxide and the vapor come out through chimneys, and then, cools gradually up to the temperature before combustion. The vapor of high temperature consumes calorific values for cooing, and the remained one is the lower heating values. The combustion gas keeps the remained calorific value, which is called the true calorific value. |
| |
| We need to know the temperature and so on during the combustion gas works before cooling. Therefore, the higher heating values is used for the simulation. The Tokyo Gas also show it, because the 13A does work just after the burning, like heating foods for cooking. |
| |
 |
| (4)-3 Sulfur in the Fuel |
| |
| It is not a gas, it is not added intentionally; however, very small quantity is sometimes included. |
| |
| The sulfer can be burnt and it may be a part of the flammable components. It produces sulfer oxide gas. It turns to sulfite and sulfuric acid when it cools and with water or vapor. |
| |
| Both of sulfite and sulfuric acid easily corrode the mechanical structures of the heating furnaces. We can omit the sulfur if we do the temperature simulation of the material. However, the sulfite and sulfuric acid are harmful to the mechanical structure of the lower temperature parts of the heat exchanging equipment. Therefore, for the practical operations, it is suggested to pay attention to the sulfur components of the fuel. |
| |
 |
| (5) Theoretical Air Volume, Theoretical Exhaust Gas Amount |
| |
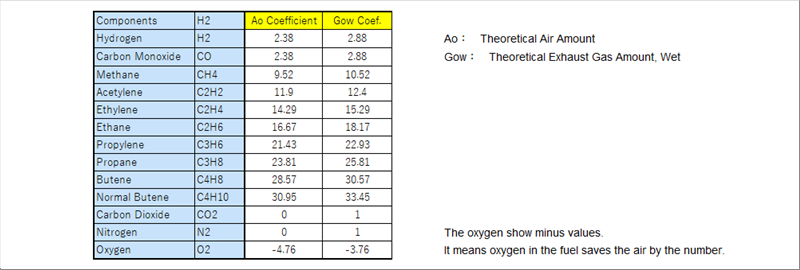
| The theoretical air amount is defined to the minimum air amount that just burns the right amount of a fuel. There are two (2) kinds of the theoretical exaust gases. It is due to "wet" or dry. The exhaust gas is very hot and contains vapor of the temperature, which is expressed "wet". |
| |
 |
| |
| Both of the air and exhaust gas amounts are calculated by the same formula of the calorific value, that is each value times each ratio. |
| |
| For the A13 of Tokyo Gas, the air amount is eleven (11) and the exhaust gas is twelve (12) for one (1) A13. |
| |
 |
| (5)-1 Air-Fuel Ratio |
| The air-fuel ratio is how many times the theoretical one. |
| |
| It shall be one (1) and more in order to avoid the incomplete combustion. The excess air shall be added to the amount of the exhaust gas. |
| |
 |
| (6) Is the Forced Convection Heat Transfer Necessary? |
| |
| Let's assume that the heating furnace is big and the amount of the exhaust gas is small. In such cases, the velocity of the exhaust gas may be small enough to ignore the forced convection. On the contrary, small heating furnaces with big quantity of exhaust gas may need it. |
| |
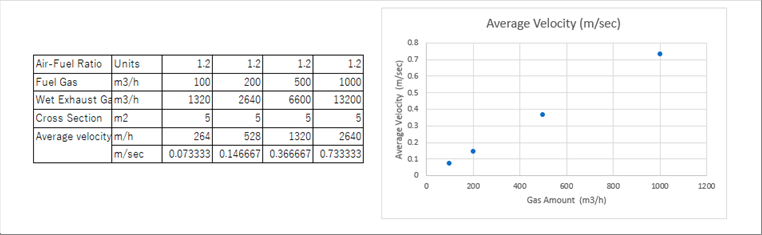
| Example: Amount of natural gas 100 to 1 000 m3/h, air-fuel ratio: 1.2, cross section of the furnace 5m2. |
| |
 |
| |
| The average velocity increases as the gas amount per a unit time increases. It may be necessary to consider the forced convection heat transfer. |
| |
 |
| (7) Chemistry of Combustion |
| |
| The basis of all above calculations is the chemical formula. Please take a look if you are interested in. |
| |
| The flammable gas and oxygen react to produce hot carbon dioxide and hot vapor. The following chemical formula shows the reaction of methane CH4. |
| |
| CH4 + 2O2 -> CO2 + 2H2O + 891 kJ |
| |
| The calorific value is shown at the ringt end of the formula. The above tables show 39.8MJ/Nm3, but is indicates 891kJ/mol. The difference of the values is due to the difference of the units. |
| |
| The mole number is not used in the usual life. However, it is one of the basis of the chamical formula. It is based on the fact that any material is made up of molecules (atoms for the metals). |
| |
| CH4 + 2O2 -> CO2 + 2H2O + 891 kJ |
| |
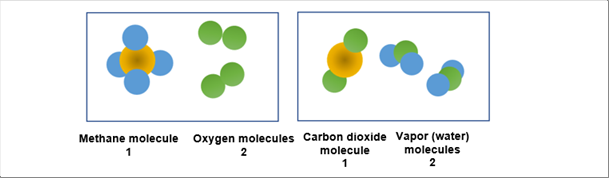
| The above chemical formula says one (1) molecule of methane and two 82) molecules of oxygen act to produce a molecule of carbon dioxide and two (2) molecules of water (vapor). |
| |
| The following pictures show the reaction. The brown balls stand for carbon atoms (diameter; 0.15nm), blue balls for hydrogen atoms (diameter; 0.12nm), and green balls for oxygen atoms(diameter; 0.14nm). |
| |
 |
| |
| The chemical formula indicate the left side box turns to the right box, generating heat. The problem is that the size of a molecule or an atom is too small. The diameter of a carbon atom is 0.15 nm (nanometer). Please note that 1 000 nm is one micron meter. Therefore, 0.15 nm is one billionth of a tennis ball. If the formula is used like this way, the heat is extremely small. |
| |
| The questing is how many are appropriate. |
| |
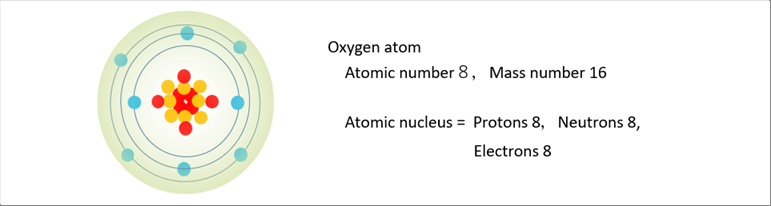
| The proposed one is the mole. It is the mass number of a material in grams. The addition of the numbers of protons and nuetrons in an atom makes the mass number. Therefore, any material of a mole contain the same numbers of molecules. The following picture shows the oxigen atom. |
| |
| An atom is made up of a nucleus and electron(s). A nucleus is made up of proton(s) and neutron(s). The mass of elections are very small, and therefore, we may be able to say that the mass of an atom is the total mass of proton(s) and neutron(s). |
| |
| Mass number �� Numbers of proton(s) �{ Numbers of neutron(s) |
| |
 |
| |
| The atom of the different material is made up of the different numbers of protons and neutrons. However, the protons and the neutrons are same. Therefore, when "grams" are added to the mass number, we can treat the same numbers of atoms for different kinds of materials. This is the definition of one (1) mole. |
| |
| One (1) mole of hydrogen = 1 g, 1 mole of oxygen = 16 g, 1 mole of carbon ��12 g. The chemical formula of the methane combustion means 1 mole of methane 12 g +4 g = 16g, 2 moles of oxygen 2 x 32 g = 64 g, total 80 g turns to 1 mole of carbon dioxide 12 g +2 x 16 g = 44 g, 1moles of vapor (water) 2 x ( 2 g +16 g ) = 36 g, total 80 g, and the heat generation is 891 kJ. |
| |
| In the volume, 1 mole of methane is 22.4 litters, 2 moles of oxygen is 2 x 22.4 = 44.8 litters, total 67.2 litters turns to 1 mole of carbon dioxide 22.4 litters, 2 moles of vapor (water) 2 x 22.4 = 44.8 litters, total 67.2 litters, and heat generation 891kJ. In the gas calculation, the unit Nm3 (normal cubic meters) are used. The above table show the values in Nm3. |
| |
| Methane 1 Nm3 heat generation 891*10^(-3)MJ / ( 22.4*10^(-3) ) Nm3 = 39.8 MJ/Nm3 |
| |
| Author: T. Oda |
| The page was prepared in Excel, and automatic html and css generation by the "excel2web". |









































