| |
 |
| |
 |
| 1 What is the Heat Treatment? |
| 2 What are differences between Water and Steel? |
| - Change of States |
| - Let Other Materials Dissolve |
| - Return to Solid with the Molten Materials |
| 3 What are differences in the Crystal Structure? |
| - Crystal Structure |
| - Crystal Structure of Steel |
| - Maximum Quantity of Carbon |
| - Quenching |
| - Reasons of the Hardening by the Heat Treatment |
| 4 Problems and Solutions of the Quenching, Tempering and Aidori (Japanese sword) |
| 5 Materials which Harden Steel other than Carbon |
| - Illustrated Reference Book for Materials |
| |
 |
| 1 What is the Heat Treatment? |
| |
| It is to heat something. So, I believe the microwave, the heat sterilization, quenching of the Japanese sword are the heat treatment. However, I would like to explain the heat treatment to the metals, such as the quenching, tempering, and Aidori for the Japanese sword. |
| |
| There are many metallic items, which are hardened by the heat treatment in addition to the Japanese sword, such as tweezers for your Gundam models, gears and bearing in the automobiles and motor cycles. |
| |
| The famous Japanese TV program, "Chico Will Scold You!", may say "The reason why the steel is hardened by the heat treatment is that the steel is similar to the water!". |
| The specialists of the materials and the heat treatment, for sure, say, "It is wrong!"; however, I believe it is easy to understand the reasons. Please let me continue as following. |
| |
 |
| 2 What ae the differences between Water and Steel? |
 |
| Change of States |
| |
| It is well known that the water changes the states due to the temperature. It is solod at zero (0) degrees C. and below. It is liquid above zero (0) degrees C., and evaporized at one hundred (100) degrees C. and higher. It is evaporized even below 100 deg. C. |
| |
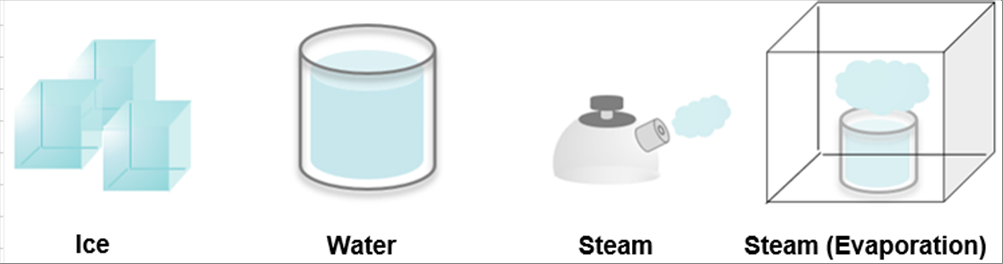 |
| |
| The steel is solid at the room temperature. It becomes liquid at about 1500 degrees C., and becomes gas at about 2800 degrees C. It evaporates even below 2800 deg. C. in vacuum. |
| |
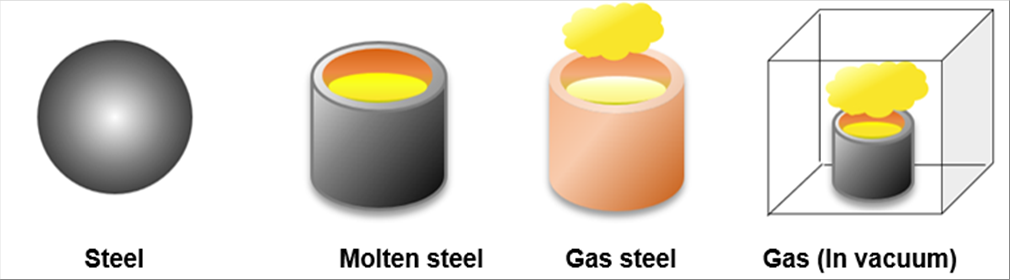 |
| |
| As seen in the above two (2) graphics, both of the water and the iron change states among solid, liquid and gas. The differences are the temperatures for the state change. The following picture shows the comparison of the temperatures of the state change. |
| |
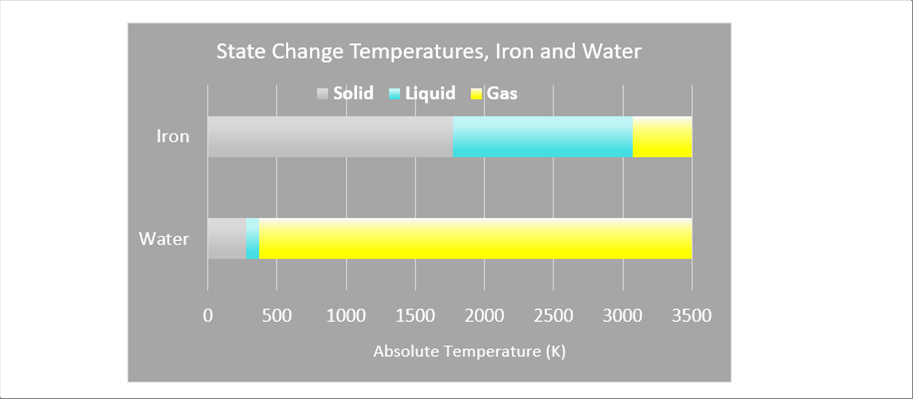 |
| |
| The gray bars show the solid, light bule the liquid, and yellow the gas. The temperatures of the state change are different between water and iron are huge. However, both materials show the state changes. |
| |
 |
| Let Other Materials Dissolve Into |
| |
| The materials cannot dissolve or mix into the water in the solide; however it allows other materials dissolve or mix into it when it is liquid. This is same to the steel. The steel let other material dissolve or mix in it when it is liquid. |
| |
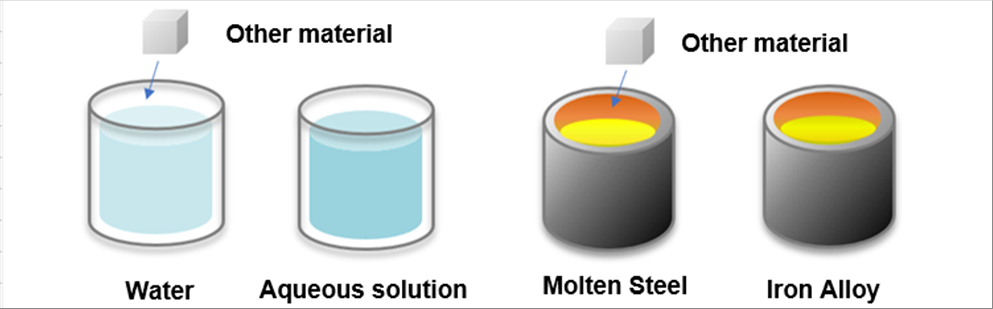 |
| |
 |
| Return to Solid with the Molten Materials |
| |
| The liquid material became solid by the cooling. It is interesting that the slow and fast cooling generate different solid materials. |
| |
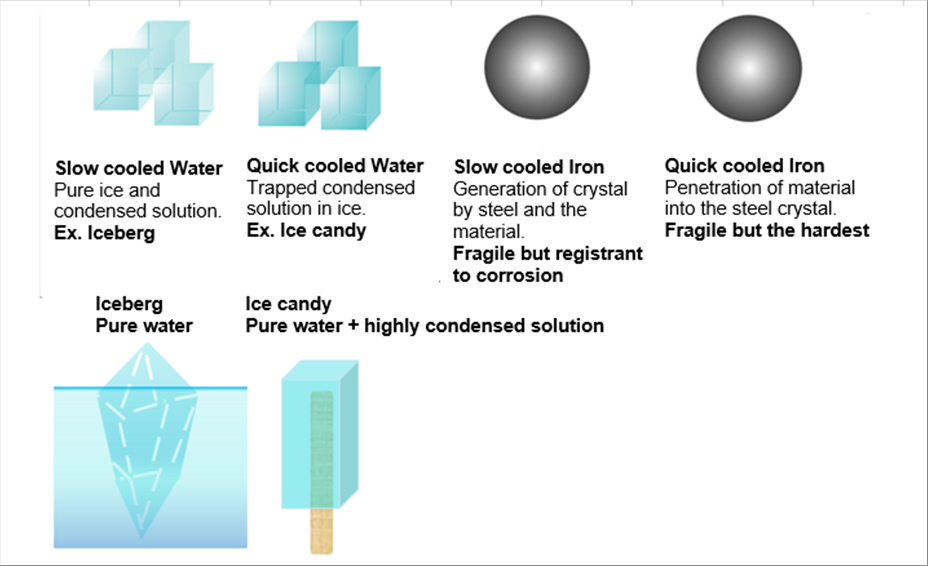
| The following pictures indicate what are obtained for both of water and steel in the slow and in the fast cooling. |
| |
 |
| |
| The solution feezes below zero (0) degrees C. The more quantity of the molten material shows the lower freezing temperature. |
| |
| The solution freezes at a temperature. As the ice becomes bigger, the concentration of the remaining solution becomes thicker. Therefore, the lower temperature is necessary for the remaining one. The slowly cooled water becomes pure water ice and the remaining concentrated solution at minus five (-5) deg. C. This is similar to the iceberg and the surrounding sea water. |
| |
| In the mean time, in the case of the quick cooling, the solution is trapped among the ice and there is no remaining solution. It is similar to the ice candies. |
| |
| The molten steel, where other material dissolves, becomes solid in the room temperature. It becomes the combination of the steel crystal and the crystal made of both of steel and the material. In the case of quick cooling, no such combination is seen but the trapped material crystal in the steel crystals. |
| |
| In the Terminator 2, the indispensable liquid type of Terminator T-1000 was finally destroyed in the molten steel. The molten one will be cooled some time. The usual one generates the combined crystal when it is slowly cooled. However, even after the slow cooling, the liquid metal might remain liquid, and it might come out of the steel to regenerate the T-1000. Was I alone to worry about it? |
| |
 |
| Cryatal Structure |
| |
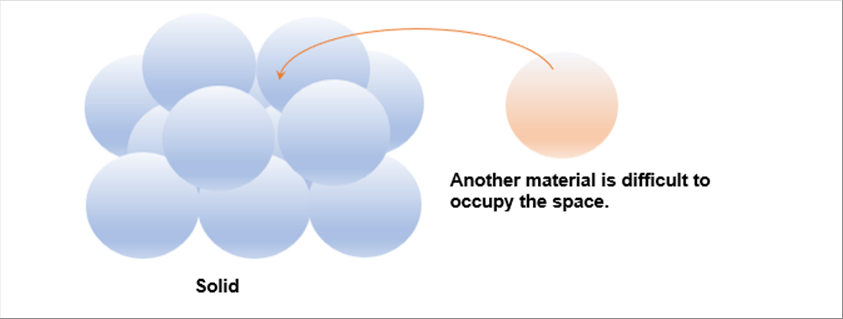
| Both of water and steel, the minimum units, whch are molecular for water and atom for steel, are lining up with small vibration. (The vibration stops at abtolute zero degree.) As the space among the atoms of the steel is few, it is, I think, difficult to add other atom among there. |
| |
 |
| |
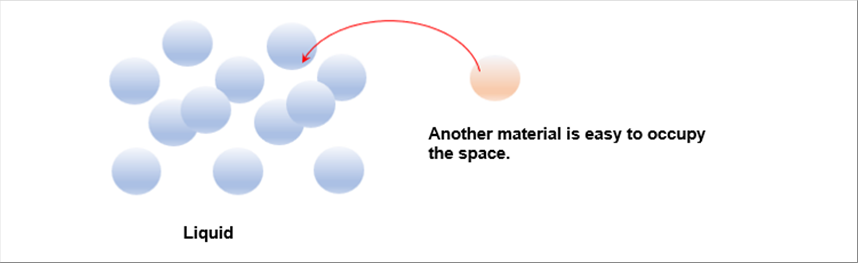
| The liquid like water can accept other material in the space among the molecules. |
| |
 |
| |
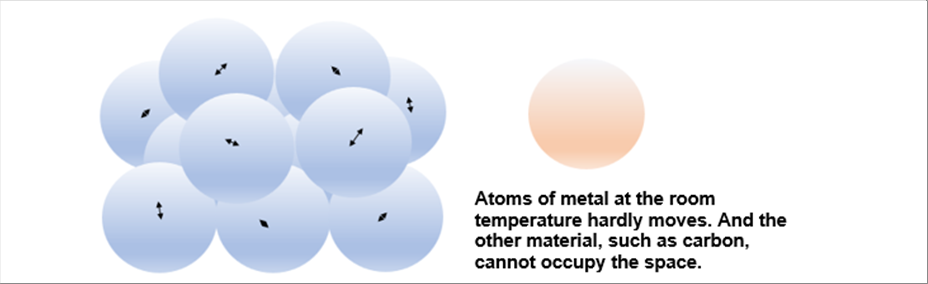
| The metal atoms vibrate at the room temperature. However, the vibration is smaller than the size of the atom. Therefore, the other atom cannot come to the space. |
| |
 |
| |
| If you paint carbon and hammer the Japanese sword at the room temperature, nothing will happen. Because there is few distance for the metal atom to accept the carbon. |
| |
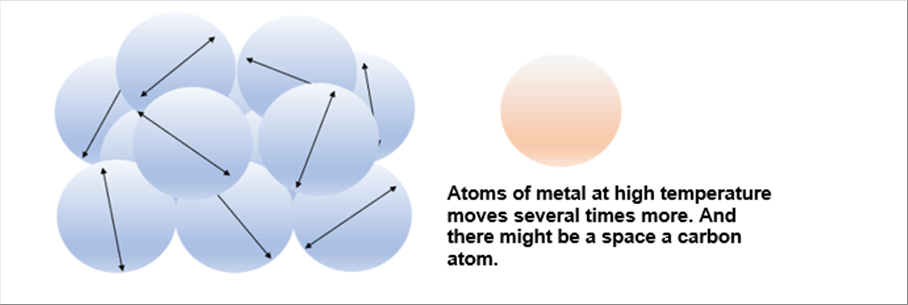
| The liquid metal at the high temperature can move more. It is much smaller than that of water; however, it may reach the same size the atom. |
| |
 |
| |
| The metal atoms can move more at the high temperature than that at the room temperature. It might be easier for the other material, such as carton, to penetrate. |
| |
| We have been seeing the Japanese sword as an example why the steal becomes harder after the heat treatment. The swordsmith does the job with the solid sword. I am sure the job is harder than the liquid ones, as the space is narrower. They say that "Charcoal preparation takes 3 years, pair hammering takes 10 years". The charcoal preparation is to obtain the even temperature for heating. It is said that the material of a Japanese sword is heated and hammered, then heated and hammered again and again. |
| |
 |
| Crystal Structure of Steel |
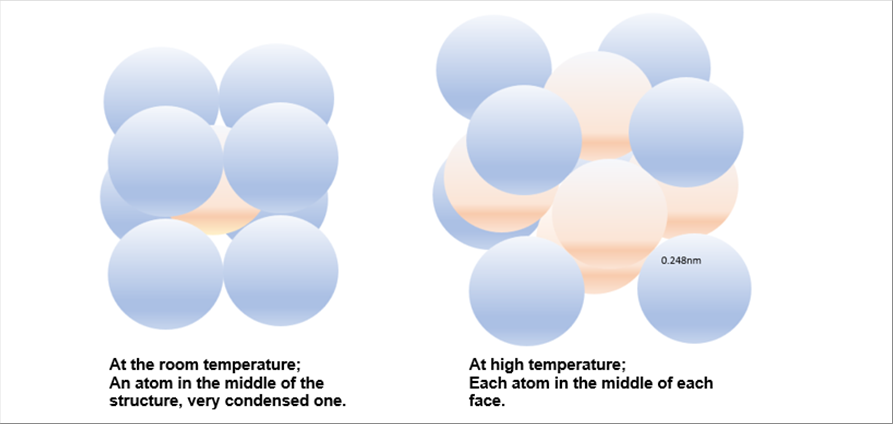
| |
| The crystal structure changes as the temperature rises. At the room temperature, there is an atom in the middle of the structure. At the high temperature, there is an atom in each face of the structure. The following pictures show the illustrated one. The "middle" atom is indicated in the different color for easy understanding. |
| |
 |
| |
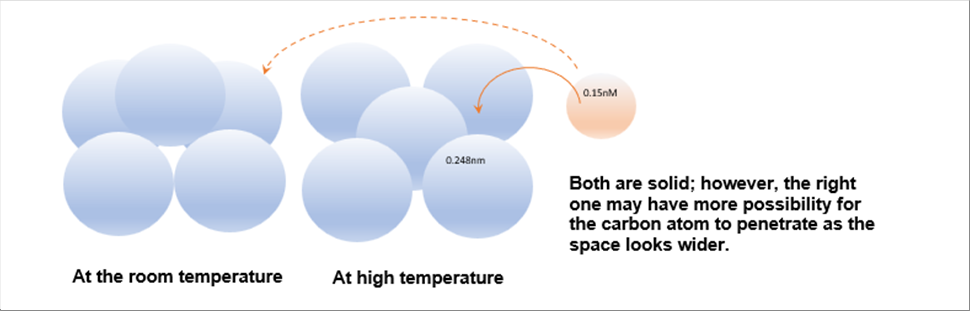
| During the heat treatment process, it is to add other atom like carbon in such structure. The following pictures show the bottom layer of the structures with a carbon atom. Different from the above ones, the blue ones are iron atoms and the orange one indicate carbon. The sizes of the iron and the carbon are 0.248 nm and 0.15 nm respectively. |
| |
 |
| |
| Both of the structures are "condensed"; however, the high temperature one has more space and therefore it has more possibility to accept the carbon atom. |
| |
| As seen in before, in addition to the crystal structure, the movable distance of the atom at high temperature is longer. Taking the two (2) conditions into account, it could be possible for a carbon atom to get into the iron atom structure. |
| |
 |
| Maximum Quantity of Carbon |
| |
| Now, we know the iron can accept the carbon. However, as seen above, it is not easy. Therefore, it is, for sure, that the maximum quantity does exist. Practically speaking, we use the steel with seven percent (7 %) and less carbon content. So, we can say that the maximum carbon content is 7%. It is called "steel iron", which contains carbon up to two (2) %, and "cast iron", from 2 % to 7 %. |
| |
| The history of steel is very long, and our forerunners had been researched and developed many things. It is now well known what and how the steel structure is with the carbon and temperature. Please see those works if you are interested in. Please let me omit to repeat the same thing in this article. |
| |
 |
| Quenching |
| |
| After capturing carbon into iron, when we cool the steel slowly, the majority of steel re-gain the original iron structure and the iron close to carbon become the compound of the carbon. |
| |
| The Japanese sword cannot be left as is but be put into the water as hot condition for quick cooling. The quick cooling is called the quenching. The quenching is one of the important processes for the steel products to finish.�B |
| |
| The atoms are fixed when the steel is quickly cooled by the water. The size of the carbon atom is about sixty (60) % of the iron one. So, when the steel is quickly cooled, the carbon aton cannot move due to the size. For example, a rice grain drops to the bottom of the box, which is filled with ping ponb balls. It is because the sizer of a rice grain is much smaler than that of the ping pong balls. |
| |
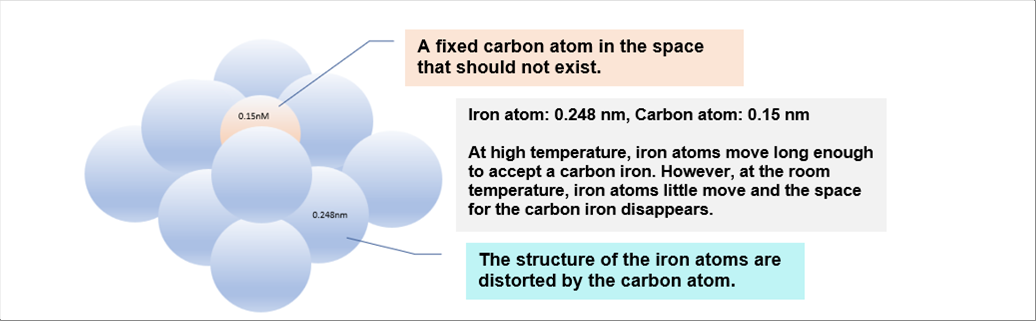
| At the room temperature, there is a carbon atom among the iron ones. It intervenes the structure of iron atoms, and therefore, it generates distortion in the structure. The "distortion" is the strain. And the strain is equal to the related stress. So, the distorted state means the forced one. (Please refer the mechanics of material for the stress and the strain.) |
| |
 |
| |
| The picture above illustrates the structure of the iron and the carbon at the room temperature. A carbon atom is fixed among the iron ones in the place that should not exist. It was OK at the high temperature, though. |
| |
| The reason why the quick cooling is essential is to avoid the generation of the compound between the carbon and the iron. Because, the compound is generated due to the slow cooling. The importance of the quenching is that the velocity of the cooling shall faster than that of the generation of the compound. |
| |
| The Japanese sword is quickly cooled by the water. The thickness of the blade is very thin, and therefore, the cooling speed of the whole blade can be almost same. So, the quenching of the blade of the Japanese sword can be considered homogeneously. |
| |
 |
| Reasons of the Hardening by the Heat Treatment |
| |
| The famous Japanese TV program, "Chico Will Scold You!", may say "The reason why the steel is hardened by the heat treatment is that the steel is similar to the water!". It means that the structure of the iron atoms at the high temperature can accept some carbon ones. And then, the quick cooling, which is quenching, is applied to fix the carbon atoms there at the room temperature. The swordsmith needs a lot of times to do the process. I hope you may understand the reason in someway. |
| |
 |
| 4 Problems and Solutions of the Quenching, Tempering and Aidori (Japanese sword) |
| |
| The carbon steel is distorted by the quick cooling and therefore it is fragile. It is easy to crack when a shock load is applied. The reason of the distortion (strain) is that there are some areas with the incomplete structure change or the incomplete precipitation. Due to the distortion (strain), it may even be broken after some time with no load. Even it does not crack, it is very brittle. In order to improve and to solve these phenomena, the tempering is applied. |
| |
| The tempering shall be applied within one (1) or two (2) hours after the quenching in order to avoid the natural cracking. It is to heat the material up to about several hundred degrees C. and keep the temperature for the defined duration. The carbon steel keeps solid during the tempering, the incompletion of structure change progresses and the precipitation of the captured materials also proceeds, thus the brittleness is solved. Thanks to the tempering, the hardness is same as before, and the toughness recovers. |
| |
| It is said that the swordsmith of the Japanese sword does not apply the tempering, but they apply "Aidori", which is keep the temperature at about two hundred (200) degrees C. The objective is same as the tempering, that is to guarantee the toughness. |
| |
 |
| 5 Materials which Harden Steel other than Carbon |
| |
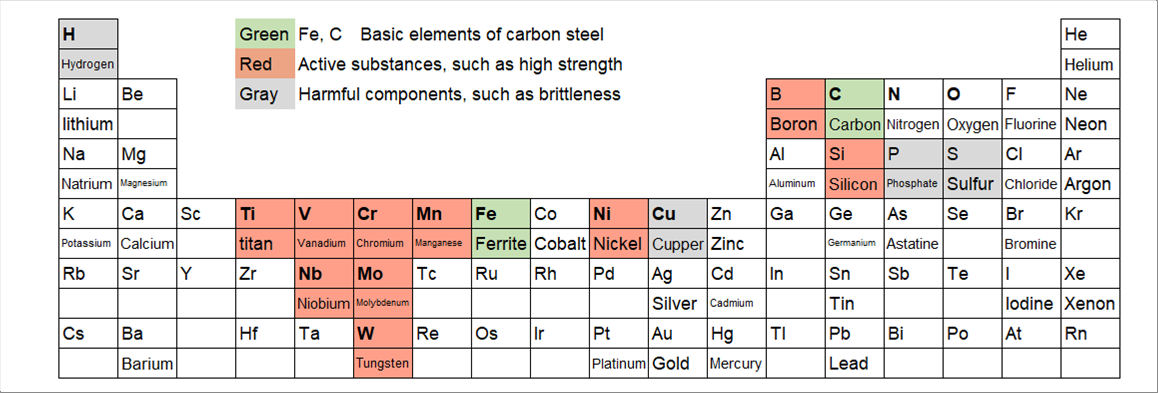
| in addition to the carbon, there are many materials that harden the iron. There are also many materials, which makes iron brittle. The following picture is the part of the Periodc Table with thre (3) colors. |
| |
| (Names of the elements are limited to the usual ones, which we see.) |
| |
 |
| |
| Green: Basic elements. Iron Fe and Carbon C are the ones. |
| Red: Active substances. It is known as the useful alloy components for higher strength. |
| Gray: Harmful components. It is known due to harm such as increasing brittleness. |
| |
| The distibution of the Reds and the Grays are not scatteed but grouped in the Periodic Table. |
| |
| There is a group of seven (7) Reds left to the basic element of Fe. They are Titanium Ti, Vanadium V, Chromium Cr, and Manganese Mn. Another Red one to the right side of the Fe is Nickle Ni. Boron B and Silicon Si are next to the other basic element of Carbon C. |
| |
| The harmful component, Cupper Cu is next to the active one Nickel NI. Other harmful ones, Phosphate P and Sulfur S are also next to the active one Silicon Si. |
| |
 |
| Illustrated Reference Book for Materials |
| |
| We see some of the elements, such as ferrite, carbon, and sulfur. However, we seldom see the other materials, such as vanadium, boron, niobium, and so on. If you are interested in the materials, it is recommended to see the elements in the illustrated reference book. I own one. It is interesting to me to see the crystal, color, and so on. |
| |
| Author: T. Oda |
| Preparation in Excel, automatic html / css generation by the "excel2web". |
| |























































